|
|
DNA as a scaffold for chromophore assembly |
|
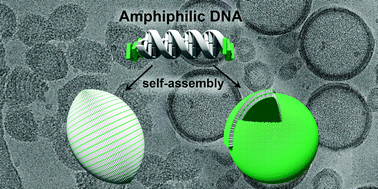
Supramolecular Assembly of DNA-Constructed Vesicles
The self-assembly of DNA hybrids possessing tetraphenylethylene sticky ends at both sides into vesicular architectures in aqueous medium is demonstrated. Cryo-electron microscopy reveals the formation of different types of morphologies from the amphiphilic DNA-hybrids. Depending on the conditions, either an extended (sheet-like) or a compact (columnar) alignment of the DNA hybrids is observed. The different modes of DNA arrangement lead to the formation of vesicles appearing either as prolate ellipsoids (type I) or as spheres (type II). The type of packing has a significant effect on the accessibility of the DNA, as evidenced by intercalation and light-harvesting experiments.
S. Rothenbühler, I. Iacovache, S.M. Langenegger,
B. Zuber, R. Häner
Nanoscale 2020, 12,
21118-21123
|
|
 |
|
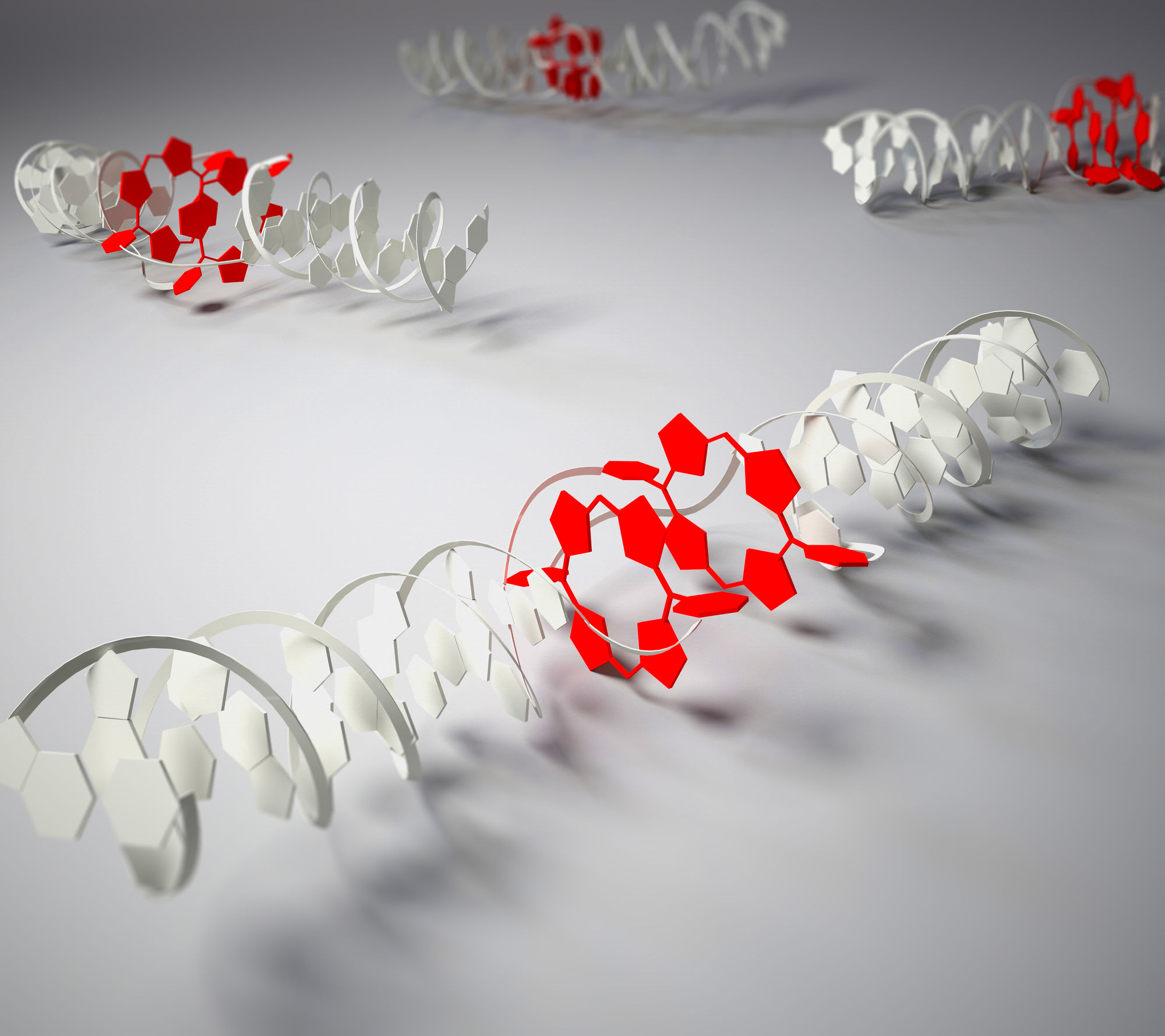
Assembling Multiporphyrin Stacks Inside the DNA Double
Helix
Double stranded DNA hybrids containing up to four consecutive,
face-to-face stacked porphyrins are described. Multiple
porphyrins are well accommodated inside the DNA scaffold
without disturbing the overall B-DNA structure. UV/vis,
fluorescence, and CD spectroscopy demonstrate the formation of
porphyrins H-aggregates inside the DNA double helix and
provide evidence for the existence of strong excitonic
coupling between interstrand stacked porphyrins. The findings
demonstrate the value of DNA for the controlled formation of
molecularly defined porphyrin aggregates.
M. Vybornyi, A.L. Nussbaumer,
S.M. Langenegger, R. Häner
Bioconjugate
Chem. 2014, 25,
1785-1793
|
|
 |
|
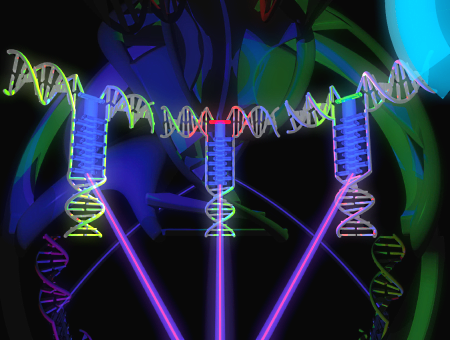
A modular LHC built on the DNA
three-way junction
A light-harvesting complex composed of a
π-stacked
multichromophoric array in a DNA three-way junction is
described. The modular design allows for a ready exchange of
non-covalently attached energy acceptors.
M. Probst, S. M. Langenegger, R. Häner
Chem. Commun. 2014, 50,
159-161
|
|
 |
|
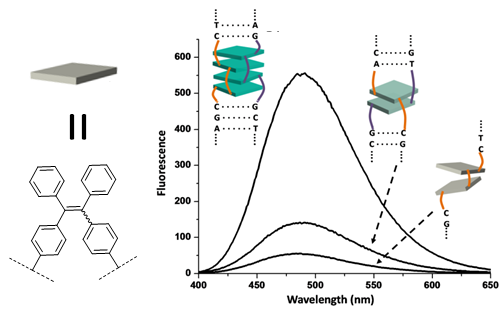
Control of aggregation-induced
emission by DNA hybridization
Aggregation-induced emission (AIE) was
studied by hybridization of dialkynyl tetraphenylethylene
(DATPE) modified DNA strands. Molecular aggregation and
fluorescence of DATPEs are controlled by duplex formation.
With increasing molecular organization, a steady increase of
fluorescence could be observed. The quantum yields of the
duplexes with four DATPEs (f = 0.31 and 0.32, respectively)
are close to the monomeric building blocks in their aggregated
states (f ~0.40).
S. Li, S.M. Langenegger, R. Häner
Chem. Commun. 2013, 49,
5835-5837
|
|
 |
|
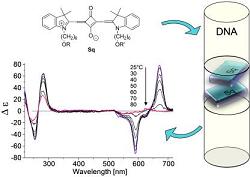
Synthesis and properties of squaraine-modified DNA
The incorporation of squaraines into DNA via the phosphoramidite approach is described. High molar absorptivity, environment-sensitive fluorescence properties and intense CD effects render squaraines valuable building blocks for DNA-based optical probes and nanostructures.
L.I. Markova, V.L. Malinovskii, L.D. Patsenker, R. Häner
Org. Biomol. Chem. 2012, 10, 8944-8947
|
|
|
|
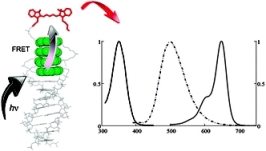
Photon harvesting by excimer-forming
multichromophores
A light-harvesting system based on a
DNA-organized oligopyrene–cyanine complex is described. Energy
transfer from the pyrene units (highlighted
green) to the cyanine dye (red
structure) was found to proceed via FRET from locally
confined excimers to the acceptor.
Oliver O. Adeyemi, Vladimir L. Malinovskii, Sarah M. Biner,
Gion Calzaferri and Robert Häner
Chem. Commun. 2012, 48,
9589-9591
|
|
 |
|
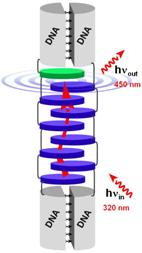
A DNA-based light harvesting antenna
An efficient mechanism for the collection of light is an essential element of natural and artificial photosynthetic systems. It involves the absorption of sunlight by a light harvesting complex (LHC) followed by excitation energy transfer (EET) to areaction center.We describe a DNA-based, light harvesting antenna which consists of an array of
π-stacked phenanthrene chromophores (the light collecting antenna, blue disks), an exciplex forming pyrene (the energy collection center, green disk) and a double helical DNA (the supramolecular frame, grey cylinders). Up to eight phenanthrenes have been used for light collection. The number of photons emitted by the phenanthrene-pyrene exciplex is proportional to the number of light absorbing chromophores.
F. Garo, R. Häner
Angew. Chem. Int. Ed. 2012, 51, 916-919
|
|
|
|
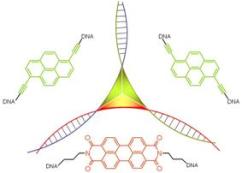
The DNA three-way junction as a mould for tripartite chromophore assembly
The DNA three-way junction (3WJ) serves as a scaffold for the molecular organization of non-nucleosidic pyrene and perylene building blocks, located at the branch point of this higher order DNA structure. The 3WJ is formed by three oligodeoxynucleotides that are partially complementary in a pairwise fashion. Two chromophores, dialkynylpyrene and perylenediimide, are positioned in the middle of the oligomers. Depending on the composition of the tripartite assembly, the constructs possess distinct spectroscopic properties, ranging from monomer or excimer fluorescence to completely quenched tripartite aggregates. The spectroscopic properties of these aggregates are largely controlled by the nature of the interstrand stacking interactions of the chromophores.
M. Probst, D. Wenger, S.M. Biner and R. Häner
Org. Biomol. Chem. 2012, 10, 755-759
|
|
|
|
Modulation of chiroptical properties by DNA-guided assembly of fluorenes
The synthesis and the incorporation of non-nucleosidic fluorene derivatives are described. The interstrand interactions lead to an additional stabilization of the hybrid and the interaction of the two chromophores results in an excimer emission. The key finding is the modulation of the chiroptical properties by a simple change in the linker size.
D. Wenger, V.L. Malinovskii and R. Häner
Chem. Commun. 2011, 47, 3168-3170
|
|
|
|
Dialkynylpyrenes: Strongly Fluorescent, Environment-Sensitive DNA Building Blocks
Two isomeric dialkynylpyrene phosphoramidites and their incorporation into oligonucleotides are described. The pyrene units closely resemble the well-known perylene bisimide dye PDI with regard to the ability to self-organize within a DNA duplex. In addition, dialkynylpyrenes exhibit significant monomer and remarkably strong excimer fluorescence. The dialkynylpyrene building blocks are promising candidates for applications in diagnostic tools, such as excimer-based molecular beacons, as well as for novel DNA-based materials with special optical properties.
H. Bittermann, D. Siegemund, V.L. Malinovskii, R. Häner
J. Am. Chem. Soc. 2008, 130, 15285-15287
|
|
|
|
TTF-Modified DNA
A non-nucleosidic tetrathiafulvalene (TTF) building block and its incorporation into DNA are described. TTF-modified oligonucleotides form stable double strands. Exciton coupling reveals a high degree of structural organization in the modified region of a hetero-duplex formed by TTF and perylene diimide-containing strands. Photo-induced electron transfer is demonstrated by fluorescence quenching in TTF/pyrene modified hetero-hybrids. The results presented are important for the development of redox-active, oligonucleotide-based diagnostics or optical sensors.
N. Bouquin, V.L. Malinovskii, X. Guégano, S.X. Liu, S. Decurtins and
R. Häner
Chem. Eur. J. 2008, 5732-5736
|
|
|
|
Highly efficient quenching of excimer fluorescence by perylene diimide in DNA
Perylene diimide (PDI) functions as a powerful fluorescence quencher. The excimer signal present in single stranded DNA containing adjacent pyrenes is completely suppressed upon duplex formation with a PDI modified complementary strand. The efficieny of the process is attributed, at least partly, to interstrand stacking of pyrene and PDI units.
N. Bouquin, V.L. Malinovskii and R. Häner
Chem. Comm. 2008, 1974-1976
|
|
|
|
Monomeric and Heterodimeric Triple Helical Mimics
We developed artificial triple helical structures with oligonucleotides containing non-nucleosidic phenanthrenes and pyrenes. The polyaromatic building blocks, which are used as connectors between the Hoogsteen strand and the Watson-Crick hairpin, lead to a significant stabilization of intramolecular triple helices. Description of the relative orientation of pyrene building blocks (left) is rendered possible by the observation of exciton coupling in the circular dichroism spectra. Furthermore, the formation of heterodimeric triple helical constructs is explored. Again, the polyaromatic residues are found to have a positive effect on the stability of these structures (right). The results described are important for the design and construction of nucleic acid based triple helical architectures. Moreover, they will aide developing of analogues of biologically important, naturally occurring triple helical structures.
I. Trkulja, R. Häner
J. Am. Chem. Soc. 2007, 129, 7982-7989
|
|
|
|
Helical Arrangement of Interstrand Stacked Pyrenes in a DNA Framework
A self-organizing system is described, which is composed of two oligopyrene strands leading to the formation of an interstrand helical stack embedded in a double stranded DNA. Helical organization takes place in a hybrid containing fourteen consecutive achiral pyrene building blocks. Based on structural information obtained by CD-spectroscopy, a model of a right-handed helical stack of pyrenes was described. The findings are important for the design of artificial molecular double stranded helices for applications in nanotechnology.
R. Häner, F. Samain, V.L. Malinovskii
Chem. Eur. J. 2009, 15, 5701-5708
V.L. Malinovskii, F. Samain, R. Häner
Angew. Chem. Int. Ed. 2007, 46, 4464-4467 |
|
|
|
Non-nucleosidic, non-hydrogen bonding DNA building
blocks
We have developed various non-nucleosidic building blocks, which can be incorporated into DNA. Phenanthrene or phenanthroline derivatives with flexible linkers act as non-hydrogen bonding nucleobase surrogates. Complementary oligonucleotides containing such modifications in opposite positions form stable duplexes. The aromatic or heteroaromatic hydrocarbons ‘pair' with each other through interstrand stacking interactions. Such building blocks can also be used for stabilization of duplex DNA containing an abasic site.
S.M. Langenegger, R. Häner
Chem. Biodiv. 2004, 1, 259-264
A. Stutz, S.M. Langenegger, R. Häner
Helv. Chim. Acta 2003, 86, 3156-3163 |
|
|
|
|