|
|
Oligoarenotides |
|
|
 |
Integrating DNA Photonic Wires into Light-Harvesting
Supramolecular Polymers
A 2,7-disubstituted phosphodiester-linked
phenanthrene trimer forms tubular structures in aqueous media.
Chromophores are arranged in H-aggregates. Incorporation of
small quantities of pyrene results in the development of
light-harvesting nanotubes in which phenanthrenes act as
antenna chromophores and pyrenes as energy acceptors. Energy
collection is most efficient after excitation at the
phenanthrene H-band. Fluorescence quantum yields up to 23 %
are reached in pyrene doped, supramolecular nanotubes.
M. Kownacki, S. M. Langenegger, S.-X. Liu, R. Häner
Angew. Chem. Int. Ed.
2019,
58, 751–755
|
|
 |
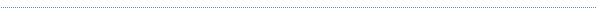 |
| |
Light-Harvesting Nanotubes Formed by
Supramolecular Assembly of Aromatic Oligophosphates
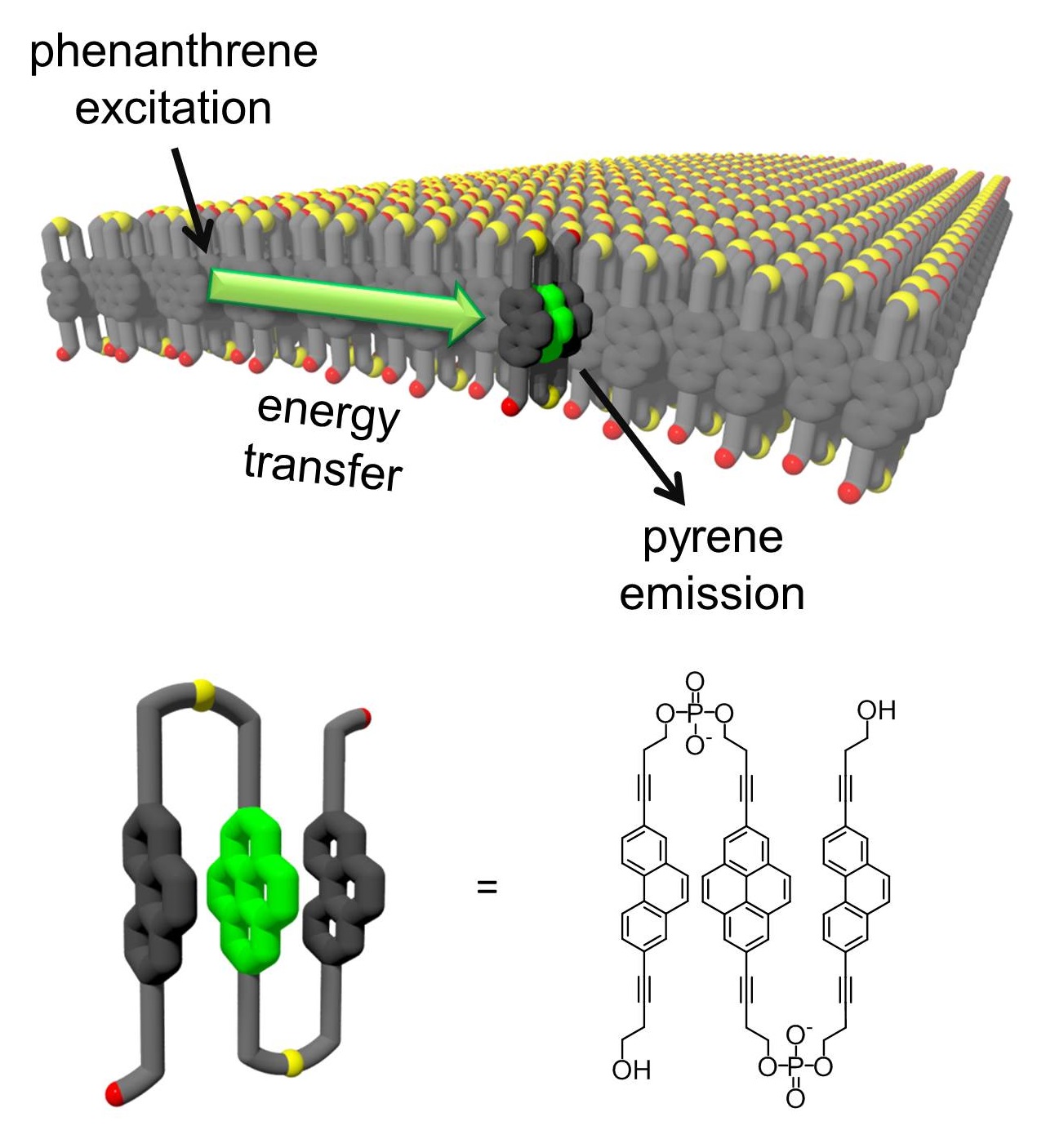
A 2,7-disubstituted phosphodiester-linked
phenanthrene trimer forms tubular structures in aqueous media.
Chromophores are arranged in H-aggregates. Incorporation of
small quantities of pyrene results in the development of
light-harvesting nanotubes in which phenanthrenes act as
antenna chromophores and pyrenes as energy acceptors. Energy
collection is most efficient after excitation at the
phenanthrene H-band. Fluorescence quantum yields up to 23 %
are reached in pyrene doped, supramolecular nanotubes.
C.D. Bösch, S.M. Langenegger, R. Häner
Angew. Chem. Int. Ed.
2016,
55, 9961-9964
|
|
 |
|
| |
|
DNA-Grafted
Supramolecular Polymers
DNA-grafted supramolecular polymers combine
the intrinsic properties of self-assembled materials with the
potential of sequence-specific functionalization. The
formation of helical ribbon structures is driven by pyrene
stacking interactions and enables the controlled arrangement
of oligonucleotide strands along the edges of the
supramolecular polymers.
Y. Vyborna, M. Vybornyi, A. Rudnev, R. Häner
Angew. Chem. Int. Ed., 2015, 54,
7934-7938
Y.
Vyborna, M. Vybornyi, R. Häner
J. Am. Chem. Soc. 2015, 137, 14051-14054
|
|
 |
|
| |
|
Long Distance EET in Light-Harvesting
Supramolecular Polymers
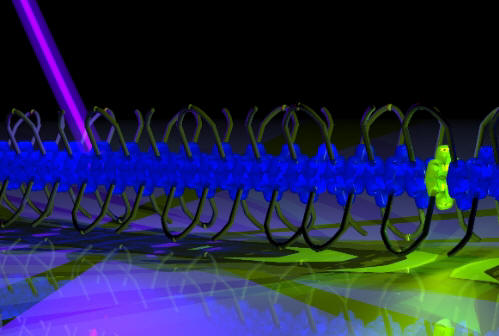
Collect and deliver: Pyrene-doped
supramolecular polymers assembled from short amphiphilic
phenanthrene oligomers exhibit light-harvesting properties.
Energy transfer from the light absorbing phenanthrenes to the
pyrene acceptor molecules proceeds in a highly efficient way
over distances well beyond 100 nm, suggesting a quantum
coherent mechanism.
C.B. Winiger, S. Li, G.R. Kumar, S.M. Langenegger, R. Häner
Angew. Chem. Int. Ed., 2014, 53,
13609–13613
|
|
 |
|
|
| |
|
Formation of Two-Dimensional Supramolecular
Polymers by Amphiphilic Pyrene Oligomers
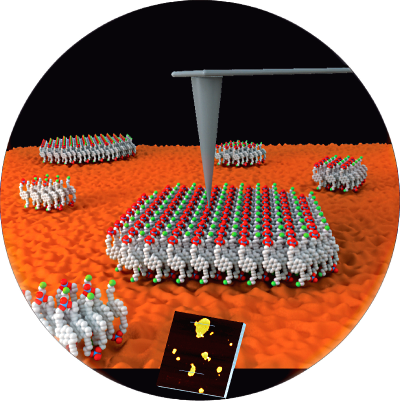
Reading the bands: Amphiphilic pyrene
trimers self-assemble into two-dimensional, supramolecular
polymers in aqueous medium. Folding and aggregation processes
are accompanied by simultaneous development of J- and H-bands
and significant changes in the fluorescence properties. The
formation of sheet-like nano-structures is confirmed by AFM.
M. Vybornyi, A. Rudnev, R. Häner
Chem. Mater., 2015,
27, 1426-1431
M. Vybornyi, A.V. Rudnev, S.M. Langenegger, T. Wandlowski, G.
Calzaferri, R. Häner
Angew. Chem. Int. Ed. 2013, 52, 11488–11493
|
|
 |
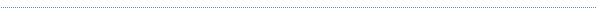 |
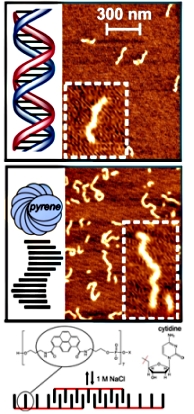
Cooperative and Noncooperative Assembly of
Oligopyrenotides Resolved by Atomic Force Microscopy
The supramolecular assembly of amphiphilic
oligopyrenotide building blocks (covalently linked
heptapyrenyl, Py7) is studied by atomic force microscopy (AFM)
in combination with optical spectroscopy. The presented study
show, that the assembly process is triggered in a controlled
manner by increasing the ionic strength of the aqueous
oligomer solution. Cooperative noncovalent interactions
between individual oligomeric units lead to the formation of
DNA-like supramolecular polymers. We also show that the
terminal attachment of a single cytidine nucleotide to the
heptapyrenotide (Py7-C) changes the association process from a
cooperative (nucleation−elongation) to a noncooperative
(isodesmic) regime. Further it was demonstrated that AFM
enables the identification and characterization of minute
concentrations of the supramolecular products, which was not
accessible by conventional optical spectroscopy.
A.V. Rudnev, V.L. Malinovskii, A.L. Nussbaumer, A. Mishchenko,
R. Häner and
T. Wandlowski
Macromol. 2012, 45,
5986–5992
|
|
 |
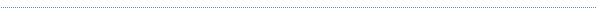 |
| |
|
|
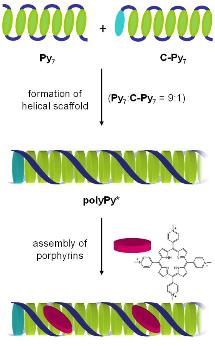
Oligopyrenotides: Chiral Nanoscale Templates for Chromophore Assembly
The formation of chiral supramolecular assemblies is an important aspect in the development of novel types of materials. DNA is well known to serve as a template for the organization of functional molecules. Among the many types of compounds investigated, cationic porphyrins take a prominent place and as a result nucleic acid - ligand interactions are well characterized. A wide variety of ligand-DNA complexes have been described, however, changes in the DNA template are limited.
We found that oligopyrenotides can serve as a chiral nanoscale template for the assembly of cationic porphyrins. The polymer-ligand aggregate resembles the well-characterized H2TMPyP-poly(dA:dT) complex. Oligopyrenotides represent valuable alternatives to DNA scaffolds with substantially different electronic properties.
A.L. Nussbaumer, F. Samain, V.L. Malinovskii, R. Häner
Org. Biomol. Chem. 2012, 10, 4891-4898
V.L. Malinovskii, A.L. Nussbaumer, R. Häner
Angew. Chem. Int. Ed. 2012,
51,
4905-4908
|
|
 |
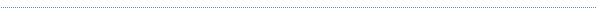 |
| |
|
|
Amplification of chirality by supramolecular polymerization of pyrene oligomers
Over the past two decades, the field of supramolecular polymers has emerged as a separate area of materials research. Structural and functional properties of supramolecular polymers largely depend on the nature of the non-covalent interactions between the individual units. We found that supramolecular polymers are formed through pi-pi stacking interactions of short pyrene oligomers. Amplification of chirality was observed by the addition of small quantities of oligomer x (chiral) to oligomer a (achiral). The described oligomers consist of 7 pyrene units linked via a flexible phosphodiester backbone. Together with other results from spectroscopic measurements like UV/Vis, fluorescence and CD spectroscopy the amplification of chirality is in strong agreement with the formation of supramolecular polymers. Further evidence for this model was found by gel electrophoresis and transmission electron microscopy (TEM).
A.L. Nussbaumer, D. Studer, V.L. Malinovskii and R. Häner
Angew. Chem. Int. Ed. 2011, 24, 5490-5494
|
|
|
|
|
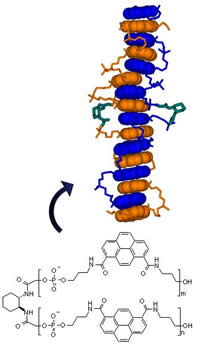
Oligopyrenotides – Abiotic, Polyanionic Oligomers with Nucleic Acid-Like Structural Properties
Oligopyrenotides, abiotic oligomers that exhibit significant structural analogies to the nucleic acids, are described. They are composed of achiral, phosphodiester-linked pyrene building blocks and a single chiral 1,2-diaminocyclohexane unit. These oligomers form stable hybrids in aqueous solution. Hybridization is based on stacking interactions of the pyrene building blocks. They show thermal denaturation/renaturation behavior that closely resembles DNA and RNA hybridization. In addition, oligopyrenotides display saltconcentration- dependent structural polymorphism. Thus, they possess a number of structural attributes that are typical of nucleic acids and therefore may serve as model systems for the design of artificial selfreplicating systems.
R. Häner, F. Garo, D. Wenger, V.L. Malinovskii
J. Am. Chem. Soc. 2010, 132 (21), 7466–7471 |
|
 |
|
| |
| |
|
|
|
|